Evolving Secondary Pharmacology: Functional, Dose–Response Safety Panels For Every Stage Of Discovery
This blog post was written by ICE Bioscience, a global contract research organization (CRO) providing preclinical bioscience platforms, focusing on new target, new assay and new technology to support in vitro biology, DMPK and in vivo pharmacology screening and profiling. Their services are available on Scientist.com.
Secondary Pharmacology: A Critical Foundation for Drug Safety Screening
In small-molecule drug discovery, unintended pharmacological activity beyond the primary target is common. Many approved drugs interact with multiple proteins, a phenomenon known as polypharmacology. While this can occasionally be therapeutically beneficial, particularly when multiple signaling pathways are intentionally targeted, it often leads to undesirable and sometimes severe adverse drug reactions.

Some of the most serious safety-related failures in drug development have been traced back to off-target effects — specific but unintended interactions with proteins not originally considered in the drug design process. These include cases such as blockade of cardiac potassium channels or activation of receptors associated with valvular disease, which have led to market withdrawals and black-box warnings.
As a result, the pharmaceutical industry has increasingly adopted secondary pharmacology screening as a standard practice. This approach involves profiling investigational compounds against a panel of targets known to be associated with clinical safety risks. The objective is to detect off-target interactions early in the discovery process — before lead optimization or candidate selection — so that potential liabilities can be designed out or deprioritized.
Safety panels are central to this process. These panels are composed of selected targets that represent key physiological systems, including the cardiovascular, central nervous, endocrine and immune systems.
Safety-44 vs Safety-77: Broader Targets, Deeper Safety Insight
The Bowes-44 panel, published in 2012, was the first widely adopted framework for secondary pharmacology screening. It assembled 44 targets across major classes — GPCRs, ion channels, enzymes, transporters and nuclear receptors — that were historically linked to clinical safety concerns. The panel was designed to be broad enough to capture common liabilities yet compact enough to be feasible in early discovery programs.

More than a decade later, industry experience and cross-company data sharing led to the development of the Safety-77 panel. This expanded set of 77 targets retains the foundation of Bowes-44 but incorporates additional targets to address gaps identified through clinical experience and regulatory feedback. In particular, Safety-77 enriches representation of under-represented families such as kinases, non-kinase enzymes and nuclear receptors, and broadens coverage of organ systems beyond the cardiovascular and central nervous system.
The evolution from 44 to 77 reflects a shift in industry perspective — from focusing narrowly on well-known safety liabilities to adopting a more comprehensive approach that anticipates a wider spectrum of off-target risks. While not yet a universal consensus, the Safety-77 framework is increasingly recognized as a more representative tool for guiding secondary pharmacology strategy across the sector.
Why Functional and Dose – Response Profiling Matter
In secondary pharmacology, assay format shapes the value of the data. While binding assays can confirm interaction, only functional assays combined with full dose – response profiling can reveal what that interaction means biologically — whether it activates, inhibits or modulates a target in more complex ways.
This distinction is critical for key target classes like GPCRs, ion channels and enzymes, where compounds may bind without triggering a functional effect, or exhibit partial agonism, inverse agonism or biased signaling. These nuances are invisible in single-point screening but may have direct implications for safety.
Why dose – response data is essential:
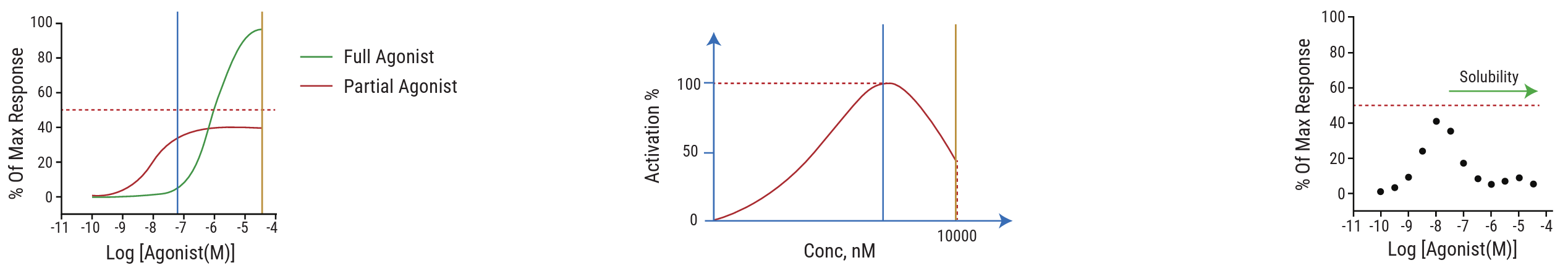
- Full vs Partial Agonism: Only concentration – response curves can reveal whether a compound acts as a full or partial agonist — an important factor when assessing risk linked to overstimulation or insufficient antagonism.
- Bell-Shaped Curves: Some targets exhibit non-linear dose – response behavior. Without full profiling, such effects can be misread or missed entirely.
- Solubility-Driven Misreads: Apparent loss of activity at higher doses may reflect solubility limits, not real pharmacology. Dose – response profiling helps separate true biological effects from assay artifacts.
By delivering both mechanistic insight and quantitative potency, functional dose – response assays support a more predictive, translationally relevant understanding of off-target risks — moving safety assessment beyond detection to informed interpretation.
Three-Tier Safety Panels for Every Stage of Discovery
ICE Bioscience offers three tiers of fully dose – response-based functional safety panels, each aligned to a specific stage of drug discovery:
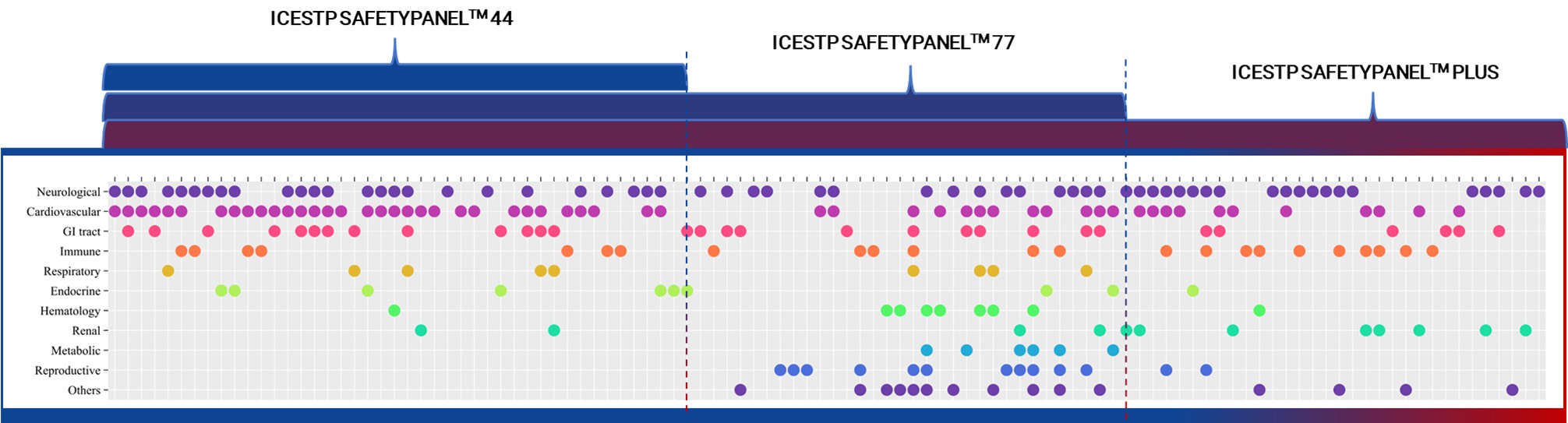
- ICESTP Safety Panel™ 44 — Designed for hit-to-lead programs, this focused panel covers core off-targets identified in legacy safety guidelines, enabling early de-risking with high efficiency.
- ICESTP Safety Panel™ 77 — Built for lead optimization, this panel reflects the latest Safety-77 framework, expanding target class diversity to support structure-activity refinement and mechanistic risk assessment.
- ICESTP Safety Panel™ Plus — Tailored for candidate selection, it screens across 108 safety-relevant targets, offering the broadest off-target coverage for final decision-making.
Physiological Kinase Assays for Greater Translational Relevance
Kinases are increasingly recognized as critical off-targets in safety assessment. As ATP-binding proteins with conserved structural domains, they are prone to unintended interactions — even from compounds not originally designed as kinase inhibitors. These off-target effects have been linked to cardiotoxicity, hematological toxicity and other dose-limiting adverse events.

Modern safety panels have responded by expanding kinase coverage. Earlier panels included few kinases, but more recent designs — like the 77-target framework — incorporate a broader range of kinases relevant to clinical safety. But target inclusion alone is not enough — assay conditions must reflect physiological reality. Many kinase assays still operate at low ATP concentrations (Km), which may exaggerate compound potency and lead to misleading safety signals.
ICE Bioscience’s safety panels not only include a broad range of clinically relevant kinases but also perform all kinase assays under 1 mM ATP conditions. This ATP concentration closely mimics intracellular levels, ensuring that only compounds with true high-affinity interactions are flagged.
Setting the Standard in Functional Safety Screening
With the ICESTP Safety Panel™ series, ICE Bioscience offers the broadest range of fully functional secondary pharmacology panels available today — from the focused 44-target panel to the expanded 77-target panel aligned with recent industry recommendations, and the extended Plus version covering 108 diverse safety-relevant targets.
While the Safety-77 framework represents an important advance, the field continues to evolve and there is not yet full consensus on the definitive composition of a “core” panel. That is why flexibility matters. All ICESTP panels are built with full dose – response profiling from the start, and for programs with specific needs, we provide custom panel design to address therapeutic context, chemical scaffold or regulatory requirements.
Whether in hit-to-lead, lead optimization or candidate selection, ICE Bioscience enables a mechanistically informed, adaptable approach to secondary pharmacology profiling — supporting confident decisions in a space where standards are still taking shape.
References- Bowes J, et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov. 2012;11(12):909 – 922. https://doi.org/10.1038/nrd3845
- Brennan RJ, et al. The state of the art in secondary pharmacology and its impact on the safety of new medicines. Nat Rev Drug Discov. 2024;23:525 – 545. https://doi.org/10.1038/s41573-024-00942-3
- Brennan RJ, et al. Shaping secondary pharmacology panels of the future: evolving target selection criteria for safety panels. Nat Rev Drug Discov. 2025. https://doi.org/10.1038/s41573-025-01184-7
- Maciag M, et al. Enzymes in secondary pharmacology screening panels: is there room for improvement? Nat Rev Drug Discov. 2025. https://doi.org/10.1038/s41573-025-01173-w
- Jenkinson S, et al. A practical guide to secondary pharmacology in drug discovery. J Pharmacol Toxicol Methods. 2020;105:106869. https://doi.org/10.1016/j.vascn.2020.106869