How Primary Skin Cells are Advancing Dermatology Drug Discovery
This blog post was written by PromoCell, a German premier manufacturer of cell culture products that helps scientists do better research with a world-class portfolio of human primary, stem and blood cells as well as optimized cell culture media. Their services are available on Scientist.com.
Conventional preclinical models, including immortalized cell lines and animal models, often fail to reflect the complex physiology of human skin. The limitations of these models contribute to low success rates and frequent failures in later stages of drug development. The increase in FDA-approved dermatological drugs between 2012 and 2022 reflects a renewed focus on innovation in the field.1 To sustain this momentum, researchers are turning to more predictive, human-relevant in vitro models that can improve early-stage discovery, better mimic patient biology and reduce dependence on animal testing.2
The skin as a drug target
Human skin fulfills multiple physiological functions beyond its role as a protective barrier. It also acts as an immunologically active, metabolically dynamic and neuroendocrine organ.3,4 As such, it presents a highly intricate biological system for drug development.
The skin’s stratified structure, from the metabolically active basal keratinocytes to the lipid-rich stratum corneum, creates distinct microenvironments that influence compound penetration, metabolism and efficacy.5 Although useful for basic mechanistic studies, traditional 2D cell culture systems cannot capture these spatial relationships or the cell-cell communications that modulate therapeutic responses.
Moreover, skin conditions often involve multiple cell types working together. Wound healing requires coordinated responses between keratinocytes, fibroblasts and endothelial cells.6 Inflammatory skin conditions involve several cytokine cascades and immune cell infiltration.7 Aging-related skin changes affect melanocyte function, collagen production and barrier integrity.8
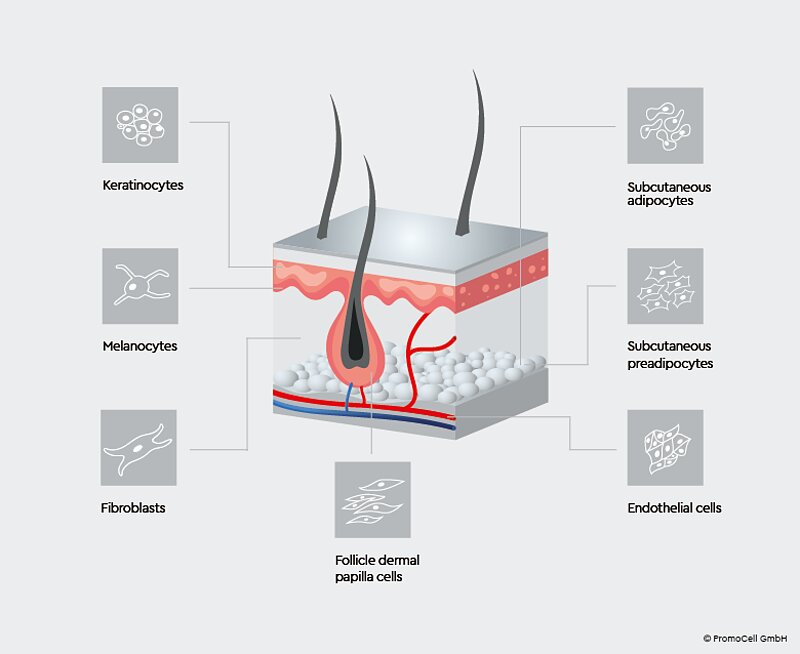 Figure 1: The skin is comprised of multiple layers, made up of different cell types. Using each cell type in research to build realistic skin models is crucial for an in-depth understanding of how the skin functions in vivo.
Figure 1: The skin is comprised of multiple layers, made up of different cell types. Using each cell type in research to build realistic skin models is crucial for an in-depth understanding of how the skin functions in vivo.
Primary human skin cells: The foundation of better models
Primary human skin cells provide distinct advantages over immortalized cell lines, making them highly valuable for drug discovery. Unlike cell lines, primary cells retain their native phenotype, metabolic behavior and responsiveness to stress.9 Therefore, they tend to yield more clinically relevant readouts in cytotoxicity and compound screening assays.
Donor variability, often regarded as a complicating factor, offers the opportunity to capture genetic, phenotypic and demographic diversity in human populations. This variability enables the identification of compounds that perform consistently across a range of individuals, while also revealing potential outliers in drug response early in development.10
Key human skin cell types driving dermatological research include:
- Keratinocytes, for barrier function studies
- Melanocytes, for pigmentation research
- Dermal fibroblasts, for wound healing and aging studies
- Hair follicle papilla cells, for alopecia research
- Dermal microvascular endothelial cells, for vascularization and drug delivery studies.
PromoCell offers a comprehensive catalog of primary human skin cells, facilitating workflows ranging from initial screening in 2D to more complex 3D co-culture systems.
2D and 3D models: Matching complexity to research questions
2D cultures of primary human skin cells remain indispensable in early-stage research. Their simplicity, reproducibility and compatibility with automation make them well-suited for high-throughput cytotoxicity screening, migration assays and compound profiling.11 However, their lack of structural complexity limits their ability to replicate tissue-level functions.
3D skin models and co-culture systems provide a more physiologically relevant context, particularly in studies where tissue architecture, cellular stratification and extracellular matrix interactions are critical.12 For example, barrier function assays require stratified epithelial models that develop proper tight junctions and lipid lamellae. Drug metabolism and compound penetration studies depend on the cellular gradients and diffusion properties found in reconstructed skin.
Advanced 3D systems are further increasing the predictive power of in vitro skin models:
- Scaffold-based models using collagen or synthetic matrices enable finer control over cellular microenvironments.13
- Organoids mimic stem cell dynamics and self-organization.14
- Bioprinting allows for precise spatial control of multiple cell types.15
- Organ-on-chip technologies integrate perfusion and mechanical stimuli, offering a more dynamic system that closely mimics the in vivo microenvironment.16
As these advanced models gain traction, they are reshaping how preclinical dermatology research addresses complex, patient-relevant disease mechanisms.
Application areas driving discovery
Wound healing
Scratch or transwell migration assays using primary dermal fibroblasts are commonly employed to assess compound effects on cell motility and tissue repair. More advanced 3D models enable researchers to study matrix remodeling, growth factor gradients and the transition from acute to chronic wound states.17,18
Inflammation
Primary keratinocytes and endothelial cells provide in vitro platforms for studying oxidative stress responses, cytokine release patterns and matrix metalloproteinase expression. Co-culture systems allow researchers to model the complex immune-epithelial interactions underlying inflammatory skin diseases.19,20
Aging and pigmentation
Senescence marker analysis in primary fibroblasts can help researchers identify anti-aging compounds, while melanocyte co-cultures are used to study skin pigmentation regulation in response to UV radiation and therapeutic interventions.21,22
Drug delivery
Reconstructed skin models with proper barrier function are used to assess permeability and compound metabolism, providing more clinically relevant data than synthetic membranes in drug delivery studies.23
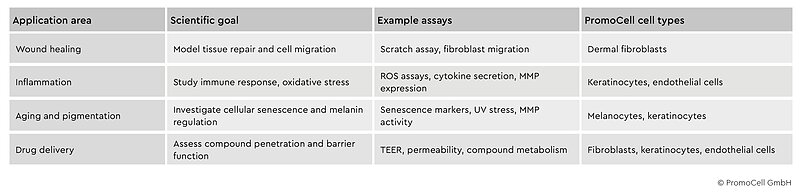 Table 1: Key application areas in dermatology drug discovery using primary human skin cells. Summary of how primary human skin cells and tissue models support diverse applications in dermatological research and drug development.
Table 1: Key application areas in dermatology drug discovery using primary human skin cells. Summary of how primary human skin cells and tissue models support diverse applications in dermatological research and drug development.
Overcoming modeling challenges
Despite their advantages, primary human skin cell models come with technical challenges. Matrix composition plays a critical role in shaping cell behavior and barrier formation. While collagen coatings are widely used, they do not fully replicate the complexity of the native basement membrane, which includes key proteins like laminin and nidogen.24 Vascularization is another significant hurdle that can limit the ability to study drug diffusion and systemic exposure. Without a functional capillary network, nutrient and oxygen gradients in 3D models can become non-physiological.25
Most in vitro skin models also lack immune components, even though immune-epithelial interactions are central to processes like inflammation and wound healing.26 Emerging co-culture approaches are beginning to incorporate immune components, opening new possibilities for studying inflammatory diseases and immunomodulatory therapies.
Finally, achieving functional barrier properties requires precise optimization of culture conditions, including media composition and differentiation protocols.23 Only high-quality primary cells under well-controlled conditions can reproduce the tight junctions and lipid organization seen in native skin.
Building smarter models for smarter discovery
Transitioning from 2D cultures of cell lines to primary human skin cells and 3D models is changing how we approach dermatological drug discovery. These systems offer the predictive power needed to identify promising compounds earlier, reduce late-stage clinical failures and ultimately bring better treatments to patients faster.
To fully realize this potential, researchers require high-quality primary cells with validated identity, functional consistency and compatibility across both 2D and 3D formats. Using well-characterized cells from diverse donors supports reproducible workflows, from initial screening through to mechanistic studies, while reflecting the variability seen in real patient populations.
Want a depper look at the latest human-relevant tools for dermatology research? Download Promocell’s infographic on modern approaches to drug discovery in dermatology and explore how human cell-based assays and 3D models are driving more predictive results.
Connect with PromoCell on Scientist.com
References
- Kamat S, Ungar B, Agarwal A, Wan J, Ross JS, Gupta R. Innovation in development of dermatologic drugs approved by the US Food and Drug Administration between 2012 and 2022. JAMA Dermatol. 2024;160(2):226-229. doi:10.1001/jamadermatol.2023.5036
- Loewa A, Feng JJ, Hedtrich S. Human disease models in drug development. Nat Rev Bioeng. 2023;1(8):545-559. doi:10.1038/s44222-023-00063-3
- Gilaberte Y, Prieto-Torres L, Pastushenko I, Juarranz Á. Chapter 1 - Anatomy and Function of the Skin. In: Hamblin MR, Avci P, Prow TW, eds. Nanoscience in Dermatology. Academic Press; 2016:1-14. doi:10.1016/B978-0-12-802926-8.00001-X
- Jin R, Luo L, Zheng J. The trinity of skin: Skin homeostasis as a neuro – endocrine – immune organ. Life. 2022;12(5):725. doi:10.3390/life12050725
- Brito S, Baek M, Bin BH. Skin structure, physiology, and pathology in topical and transdermal drug delivery. Pharmaceutics. 2024;16(11):1403. doi:10.3390/pharmaceutics16111403
- Amiri N, Golin AP, Jalili RB, Ghahary A. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Experimental Dermatology. 2022;31(4):475-484. doi:10.1111/exd.14516
- Ho AW, Kupper TS. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol. 2019;19(8):490-502. doi:10.1038/s41577-019-0162-3
- Solovev I, Sergeeva A, Geraskina A, et al. Aging and physiological barriers: mechanisms of barrier integrity changes and implications for age-related diseases. Mol Biol Rep. 2024;51(1):917. doi:10.1007/s11033-024-09833-7
- Pan C, Kumar C, Bohl S, Klingmueller U, Mann M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Molecular & Cellular Proteomics. 2009;8(3):443-450. doi:10.1074/mcp.M800258-MCP200
- Gurdasani D, Barroso I, Zeggini E, Sandhu MS. Genomics of disease risk in globally diverse populations. Nat Rev Genet. 2019;20(9):520-535. doi:10.1038/s41576-019-0144-0
- Foster NC, Hall NM, Haj AJE. Two-dimensional and three-dimensional cartilage model platforms for drug evaluation and high-throughput screening assays. Tissue Engineering Part B: Reviews. 2022;28(2):421-436. doi:10.1089/ten.teb.2020.0354
- Urzì O, Gasparro R, Costanzo E, et al. Three-dimensional cell cultures: The bridge between in vitro and in vivo models. International Journal of Molecular Sciences. 2023;24(15):12046. doi:10.3390/ijms241512046
- Weißenbruch K, Lemma ED, Hippler M, Bastmeyer M. Micro-scaffolds as synthetic cell niches: recent advances and challenges. Current Opinion in Biotechnology. 2022;73:290-299. doi:10.1016/j.copbio.2021.08.016
- Fernandes TG. Organoids as complex (bio)systems. Front Cell Dev Biol. 2023;11. doi:10.3389/fcell.2023.1268540
- Su X, Wang M, Yuan R, et al. Organoids in dynamic culture: Microfluidics and 3D printing technologies. ACS Biomater Sci Eng. 2025;11(6):3165-3181. doi:10.1021/acsbiomaterials.4c02245
- Sutterby E, Thurgood P, Baratchi S, Khoshmanesh K, Pirogova E. Microfluidic skin-on-a-chip models: Toward biomimetic artificial skin. Small. 2020;16(39):2002515. doi:10.1002/smll.202002515
- Rhee S. Fibroblasts in three dimensional matrices: cell migration and matrix remodeling. Exp Mol Med. 2009;41(12):858-865. doi:10.3858/emm.2009.41.12.096
- Berry CE, Brenac C, Gonzalez CE, et al. Natural compounds and biomimetic engineering to influence fibroblast behavior in wound healing. International Journal of Molecular Sciences. 2024;25(6):3274. doi:10.3390/ijms25063274
- Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation – a review. International Journal of Cosmetic Science. 2005;27(1):17-34. doi:10.1111/j.1467-2494.2004.00241.x
- Mohammadi MH, Heidary Araghi B, Beydaghi V, et al. Skin diseases modeling using combined tissue engineering and microfluidic technologies. Advanced Healthcare Materials. 2016;5(19):2459-2480. doi:10.1002/adhm.201600439
- Cavinato M, Waltenberger B, Baraldo G, Grade CVC, Stuppner H, Jansen-Dürr P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology. 2017;18(4):499-516. doi:10.1007/s10522-017-9715-7
- Csekes E, Račková L. Skin aging, cellular senescence and natural polyphenols. International Journal of Molecular Sciences. 2021;22(23):12641. doi:10.3390/ijms222312641
- Niehues H, Bouwstra JA, El Ghalbzouri A, Brandner JM, Zeeuwen PLJM, van den Bogaard EH. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Experimental Dermatology. 2018;27(5):501-511. doi:10.1111/exd.13531
- Cruz-Acuña R, García AJ. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biology. 2017;57-58:324-333. doi:10.1016/j.matbio.2016.06.002
- Amirsadeghi A, Jafari A, Eggermont LJ, Hashemi SS, Bencherif SA, Khorram M. Vascularization strategies for skin tissue engineering. Biomater Sci. 2020;8(15):4073-4094. doi:10.1039/D0BM00266F
- Bergers LIJC, Reijnders CMA, van den Broek LJ, et al. Immune-competent human skin disease models. Drug Discovery Today. 2016;21(9):1479-1488. doi:10.1016/j.drudis.2016.05.008